Chemistry & Chemical Reactivity| 9th Edition. Treichel/John R. Townsend/David Treichel. All-You-Can-Learn Access with Cengage Unlimited. About This Product. Help your students succeed in chemistry with the clear explanations, problem-solving strategies, and dynamic study tools of CHEMISTRY & CHEMICAL REACTIVITY. Chemistry and Chemical Reactivity (Kotz, John C.; Purcell, Keith F.) Fred Basolo. This book's first edition appeared in 1972 and has succeeded in the difficult task of staying current despite enormous changes and progress enjoyed by inorganic chemistry over the last 21 years. Download Citation. Title: Chemistry 9th Edition by Zumdahl, Steven S., Zumdahl Textbook PDF Download Author: David Kowara Subject: Chemistry 9th Edition by Zumdahl, Steven S., Zumdahl Textbook PDF Download free download.
- DOWNLOAD PDF. Tutorials Active Figures. Acknowledgments Because significant changes have been made, preparing this new edition of Chemistry & Chemical Reactivity took almost three years of continuous effort. However, as in our work on the first five editions, we have enjoyed the support and encouragement of our families and of some.
- Chemistry and Chemical Reactivity 9th Edition (eBook PDF). To you via a download link and will be sent to your email address within 5 minutes.
- Test Bank for Chemistry 9th Edition by Zumdahl,,097,instant download Chemistry 9th Edition by Zumdah pdf.
Download Test Bank for Chemistry 9th Edition by Zumdahl
Instant download and all chapter Test Bank for Chemistry 9th Edition by Zumdahl
View Sample:http://testbanksilo.com/wp-content/uploads/2017/08/Test-Bank-for-Chemistry-9th-Edition-by-Zumdahl.pdf
Test Bank for Chemistry 9th Edition by Zumdahl
Product description
This fully updated Ninth Edition of Steven and Susan Zumdahl’s CHEMISTRY brings together the solid pedagogy, easy-to-use media, and interactive exercises that today’s instructors need for their general chemistry course. Rather than focusing on rote memorization, CHEMISTRY uses a thoughtful approach built on problem-solving. For the Ninth Edition, the authors have added a new emphasis on critical systematic problem solving, new critical thinking questions, and new computer-based interactive examples to help students learn how to approach and solve chemical problems–to learn to think like chemists–so that they can apply the process of problem solving to all aspects of their lives. Students are provided with the tools to become critical thinkers: to ask questions, to apply rules and develop models, and to evaluate the outcome. In addition, Steven and Susan Zumdahl crafted ChemWork, an online program included in OWL Online Web Learning to support their approach, much as an instructor would offer support during office hours. ChemWork is just one of many study aids available with CHEMISTRY that supports the hallmarks of the textbook–a strong emphasis on models, real world applications, visual learning, and independent problem solving
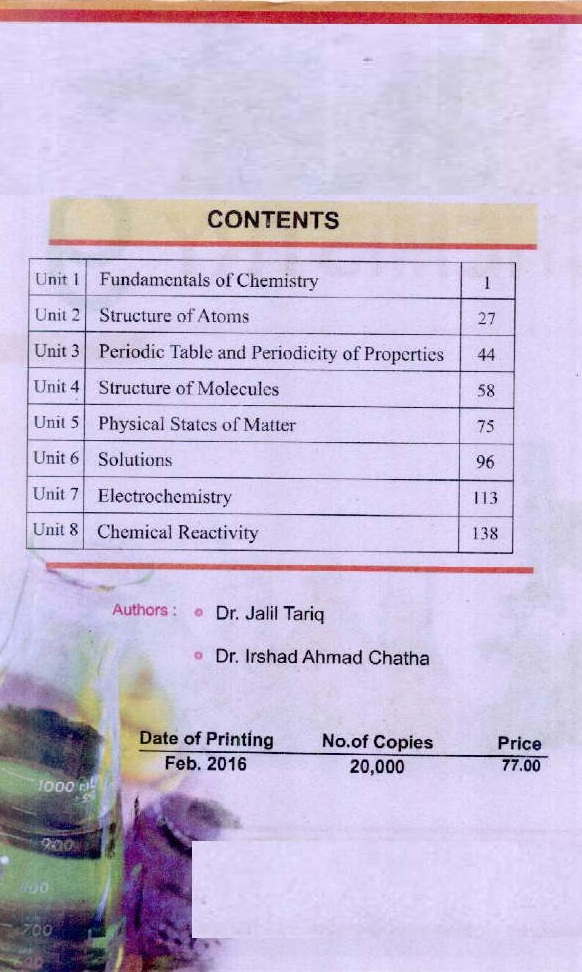
Table of contents
1. Chemical Foundations.
2. Atoms, Molecules, and Ions.
3. Stoichiometry.
4. Types of Chemical Reactions and Solution Stoichiometry.
5. Gases
6. Thermochemistry.
7. Atomic Structure and Periodicity.
8. Bonding: General Concepts.
9. Covalent Bonding: Orbitals.
10. Liquids and Solids.
11. Properties of Solutions.
12. Chemical Kinetics.
13. Chemical Equilibrium.
14. Acids and Bases.
15. Acid-Base Equilibria.
16. Solubility and Complex Ion Equilibria.
17. Spontaneity, Entropy, and Free Energy.
18. Electrochemistry.
19. The Nucleus: A Chemist’s View.
20. The Representative Elements.
21. Transition Metals and Coordination Chemistry.
22. Organic and Biological Molecules.
Appendix 1. Mathematical Procedures.
A1.1 Exponential Notation.
A1.2 Logarithms.
A1.3 Graphing Functions.
A1.4 Solving Quadratic Equations.
A1.5 Uncertainties in Measurements.
Appendix 2. The Quantitative Kinetic Molecular Model.
Appendix 3. Spectral Analysis.
Appendix 4. Selected Thermodynamic Data.
Appendix 5. Equilibrium Constants and Reduction Potentials.
A5.1 Values of Ka for Some Common Monoprotic Acids.
A5.2 Stepwise Dissociation Constants for Several Common Polyprotic Acids.
A5.3 Values of Kb for Some Common Weak Bases.
A5.4 Ksp Values at 25_C for Common Ionic Solids.
A5.5 Standard Reduction Potentials at 25_C (298K) for Many Common Half-Reactions.
Appendix 6. SI Units and Conversion Factors.
Product details
- Language: English
- ISBN-10:1133611095
- ISBN-13:978-1133611097
- ISBN-13:9781133611097
More Topic:
You will be guided to the product download page immediately once you complete the payment.
If you have any questions, or would like a receive a sample chapter before your purchase, please contact us via email : support@testbanksilo.com
Need other solution manual / test bank ?
Go to testbanksilo.com and type solution manual or test bank name you want in search box. If it not available in website, you can send email to
support@testbanksilo.com for request solution manual or test bank. We’ll reply you maximum 24 hours.
Also, you can read How to Instant download files after payment .
Relate keywords
chemistry 9th edition by zumdahl pdf
chemistry 9th edition zumdahl ebook
chemistry 9th edition zumdahl answers
chemistry 9th edition zumdahl solutions pdf
chemistry 9th edition zumdahl online
chemistry 9th edition zumdahl notes
zumdahl chemistry 9th edition chapter outlines
Save
Download Test Bank for Chemistry 9th Edition by Zumdahl
Instant download and all chapter Test Bank for Chemistry 9th Edition by Zumdahl
View Sample:http://testbanksilo.com/wp-content/uploads/2017/08/Test-Bank-for-Chemistry-9th-Edition-by-Zumdahl.pdf
Test Bank for Chemistry 9th Edition by Zumdahl
Product description
This fully updated Ninth Edition of Steven and Susan Zumdahl’s CHEMISTRY brings together the solid pedagogy, easy-to-use media, and interactive exercises that today’s instructors need for their general chemistry course. Rather than focusing on rote memorization, CHEMISTRY uses a thoughtful approach built on problem-solving. For the Ninth Edition, the authors have added a new emphasis on critical systematic problem solving, new critical thinking questions, and new computer-based interactive examples to help students learn how to approach and solve chemical problems–to learn to think like chemists–so that they can apply the process of problem solving to all aspects of their lives. Students are provided with the tools to become critical thinkers: to ask questions, to apply rules and develop models, and to evaluate the outcome. In addition, Steven and Susan Zumdahl crafted ChemWork, an online program included in OWL Online Web Learning to support their approach, much as an instructor would offer support during office hours. ChemWork is just one of many study aids available with CHEMISTRY that supports the hallmarks of the textbook–a strong emphasis on models, real world applications, visual learning, and independent problem solving
Table of contents
1. Chemical Foundations.
2. Atoms, Molecules, and Ions.
3. Stoichiometry.
4. Types of Chemical Reactions and Solution Stoichiometry.
5. Gases
6. Thermochemistry.
7. Atomic Structure and Periodicity.
8. Bonding: General Concepts.
9. Covalent Bonding: Orbitals.
10. Liquids and Solids.
11. Properties of Solutions.
12. Chemical Kinetics.
13. Chemical Equilibrium.
14. Acids and Bases.
15. Acid-Base Equilibria.
16. Solubility and Complex Ion Equilibria.
17. Spontaneity, Entropy, and Free Energy.
18. Electrochemistry.
19. The Nucleus: A Chemist’s View.
20. The Representative Elements.
21. Transition Metals and Coordination Chemistry.
22. Organic and Biological Molecules.
Appendix 1. Mathematical Procedures.
A1.1 Exponential Notation.
A1.2 Logarithms.
A1.3 Graphing Functions.
A1.4 Solving Quadratic Equations.
A1.5 Uncertainties in Measurements.
Appendix 2. The Quantitative Kinetic Molecular Model.
Appendix 3. Spectral Analysis.
Appendix 4. Selected Thermodynamic Data.
Appendix 5. Equilibrium Constants and Reduction Potentials.
A5.1 Values of Ka for Some Common Monoprotic Acids.
A5.2 Stepwise Dissociation Constants for Several Common Polyprotic Acids.
A5.3 Values of Kb for Some Common Weak Bases.
A5.4 Ksp Values at 25_C for Common Ionic Solids.
A5.5 Standard Reduction Potentials at 25_C (298K) for Many Common Half-Reactions.
Appendix 6. SI Units and Conversion Factors.
Product details
- Language: English
- ISBN-10:1133611095
- ISBN-13:978-1133611097
- ISBN-13:9781133611097
More Topic:
You will be guided to the product download page immediately once you complete the payment.
If you have any questions, or would like a receive a sample chapter before your purchase, please contact us via email : support@testbanksilo.com
Need other solution manual / test bank ?
Go to testbanksilo.com and type solution manual or test bank name you want in search box. If it not available in website, you can send email to
support@testbanksilo.com for request solution manual or test bank. We’ll reply you maximum 24 hours.
Also, you can read How to Instant download files after payment .
Relate keywords
chemistry 9th edition by zumdahl pdf
chemistry 9th edition zumdahl ebook
Chemistry And Chemical Reactivity 9th Edition Pdf Download Full
chemistry 9th edition zumdahl answers

Chemistry And Chemical Reactivity Solutions
chemistry 9th edition zumdahl solutions pdf
Chemistry And Chemical Reactivity 9th
chemistry 9th edition zumdahl online
chemistry 9th edition zumdahl notes
Chemistry 13th Edition Pdf
zumdahl chemistry 9th edition chapter outlines
Chemistry And Chemical Reactivity 9th Edition Pdf Download Pdf
Save